COVID-19 or Coronavirus Disease of 2019 is a disease caused by the SARS-CoV-2 virus (Severe Acute Respiratory Syndrome, Coronavirus-2). SARS-CoV-2 has resulted in a world-wide pandemic, which is ongoing.
More information on diagnostic testing for COVID-19 can be found below in the drop down menu.

Terminology: In conversations about the pandemic, the terms "SARS-CoV-2", "COVID-19", and "Coronavirus" are frequently used incorrectly. "SARS-CoV-2" refers to the virus itself. "COVID-19" is the disease caused by infection of SARS-CoV-2. And "Coronavirus" is a category of viruses, which includes SARS-CoV-2, that refers to the specific way that viruses look under an incredibly strong microscope.
What is COVID-19?
COVID-19 or Coronavirus Disease of 2019 is a disease caused by a newly identified virus called Severe Acute Respiratory Syndrome, Coronavirus-2 (SARS-CoV-2). SARS-CoV-2 has resulted in a world-wide pandemic, which is ongoing.
Other types of coronaviruses are known to infect humans and are one of the most frequent causes of the “common cold”.
SARS-CoV-2 is made up of ribonucleic acid (RNA, a similar material to DNA) that is surrounded by a coating made up of lipids (a type of material that does not dissolve in water and protects the RNA center of the virus) and sugars. The RNA contains all the genes the virus needs to function within a human cell.
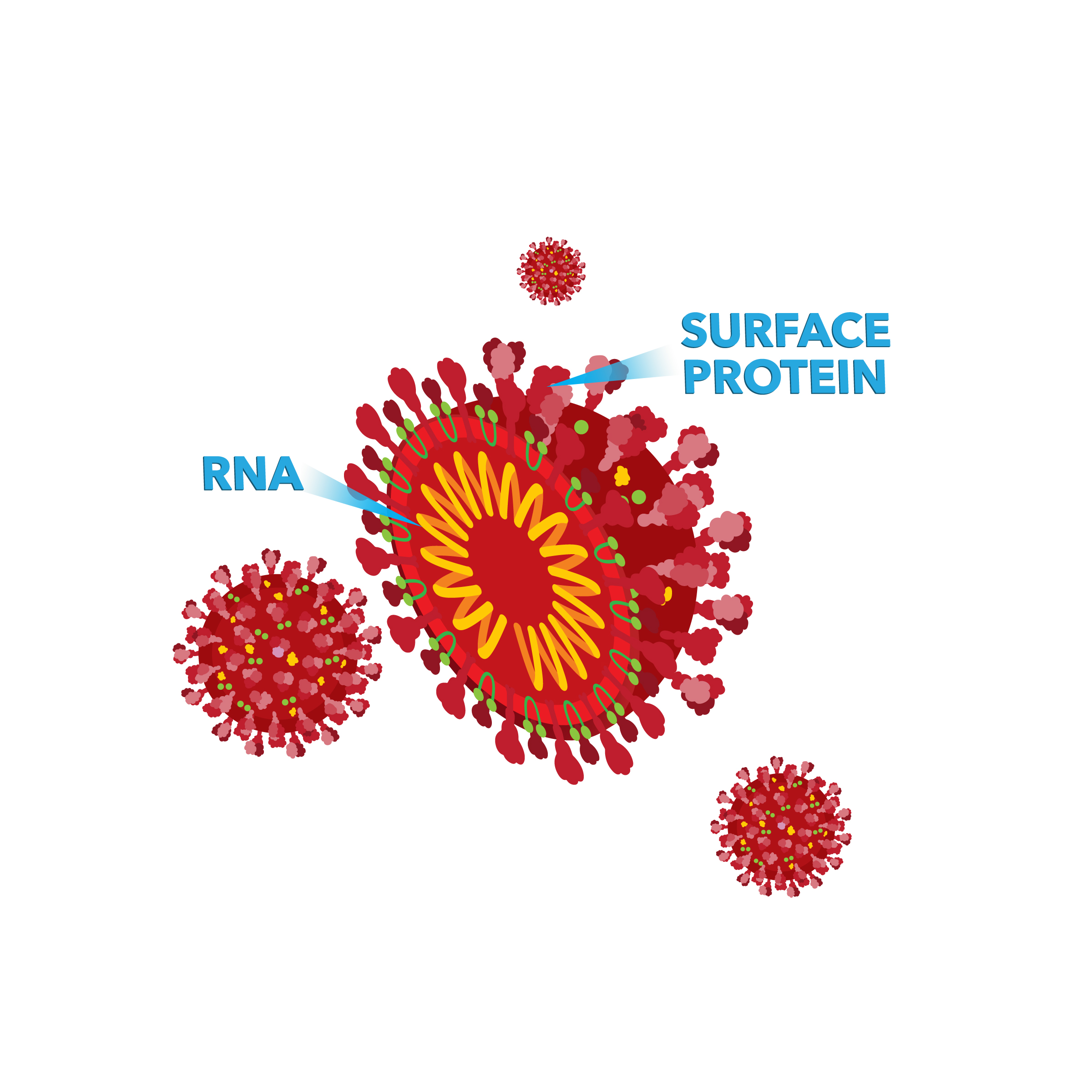
More information about COVID-19 can be found on the Centers for Disease Control and Prevention's (CDC’s) website.
How does it spread?
SARS-CoV-2 is very infectious and is spread by exposure to respiratory fluids, such as breathing in droplets that contain the virus, from people who may or may not have symptoms. More information on how SARS-CoV-2 is transmitted can be found on the CDC’s website.
What are ways to prevent exposure and spread of SARS-CoV-2?
Vaccines are injections that teach your body’s immune system to recognize and defend your body from harmful viruses and bacteria. Vaccines are the most effective measure to prevent the spread of SARS-CoV-2 and have been proven to significantly prevent severe illness, hospitalizations, and death from COVID-19. The CDC recommends that everyone eligible to receive a COVID-19 vaccine should do so.
More information on the vaccines that are currently authorized and recommended for COVID-19 in the US can be found on the CDC’s website.
In addition to vaccination, wearing a mask, social distancing measures, and quarantining after potential exposure also work in conjunction to decrease the spread of SARS-CoV-2. More information can be found on the CDC’s website.
What are the symptoms?
Symptoms can begin anywhere from 2 to 14 days after exposure to a person who is contagious or shedding the virus, with an average onset of 4-5 days. This time frame is known as the “incubation period”. The most common symptoms include cough, fever or chills, shortness of breath or difficulty breathing, fatigue and loss of taste or smell. Other symptoms can include headache, confusion, sore throat, congestion or runny nose, nausea/vomiting or diarrhea, or multisystem inflammatory syndrome in children (MIS-C), and MIS-A in adults, a rare but serious condition which causes inflammation in multiple organs. More information on COVID-19 symptoms can be found on the CDC’s website.
Regardless of vaccination status or prior infection, CDC recommends that anyone with symptoms of COVID-19 should get tested and follow the advice of your healthcare provider. Contact your local or state health department to find a testing location near you.
The severity of symptoms can range from asymptomatic infection (no symptoms) to mild disease similar to the common cold to very severe respiratory or lung disease that can require mechanical ventilation support (a type of life support where a machine, the ventilator, takes over the work of breathing for a person who is unable to breathe for themselves) and may lead to death. Why some people develop more severe disease is not completely understood; however, people at increased risk for severe illness include older adults, pregnant or recently pregnant individuals, and people with chronic medical conditions such as diabetes, heart disease, or cancer. Unfortunately, the COVID-19 pandemic has highlighted underlying health disparities in the US as COVID-19 has been shown to unequally affect underserved populations. Some people may develop long-term symptoms after infection (called “long haulers” or “long COVID”), yet these long-term effects and who is at risk for developing them are still not completely understood.
What is the infection and vaccination rate?
The pandemic continues to be a major cause of severe infection and disease throughout the world, as well as in unvaccinated people in the US. You can find up-to-date information about infection rates and levels of vaccination in the US with the CDC COVID Data Tracker.
Fluid samples collected from nasal swab or saliva can be analyzed via viral tests that detect the presence of SARS-CoV-2 RNA. Viral tests can be either molecular tests (also referred to as Nucleic Acid Amplification Tests or NAAT) or antigen tests.
Molecular tests, or Nucleic Acid Amplification Tests (NAAT), for COVID-19 can use many types of testing technology, such as: reverse transcriptase polymerase chain reaction (RT-PCR), transcription mediate amplification, CRISPR (a type of technology that can be used to edit genes), or Next-Generation Sequencing (NGS) - you can read more about how NGS technology works on our Patient Resources for Cancer page.
Alternatively, an antigen test, which directly detects a piece of the coating of the virus, can be used, but is generally less accurate than molecular tests. More information on these test types is listed below.
How Do They Work?
Molecular tests can detect the presence of all or some of the virus’ RNA in a person’s sample. Molecular tests are so sensitive that they can detect even a small amount of viral RNA if it’s present in the sample.
Routine molecular tests will detect all currently recognized variant strains. However, specific identification of SARS-CoV-2 strains detected in samples for public health surveillance purposes requires another type of testing method (see “What About the Variants?” below).
How does PCR work? PCR essentially makes billions of copies of small strands of RNA that allows the targeted areas to be detected using a variety of instruments. It is so sensitive that it can detect just a few copies of a specific sequence in a sample. PCR requires that the sequence of the targeted RNA be known and that the sequence be unique to the intended target.
How Long Do They Take?
Results are usually reported in 1-3 days in most instances but may be available in as little as an hour for select tests.
More information on molecular diagnostic tests for COVID-19 can be found on the CDC’s website.
How Do They Work?
Antigen tests detect protein fragments on the surface of the SARS-Cov-2 virus and a positive result implies an ongoing infection.
Antigen tests are most accurate in individuals who have been experiencing symptoms for fewer than 5 days and have a high amount of SARS-CoV-2 (i.e., high viral load) in their sample. Compared with the molecular diagnostic tests (described above), antigen tests are generally less “sensitive”, meaning they may not detect low levels of virus that are present. While antigen tests are generally less sensitive, they still play an important role in monitoring infection and will provide the most benefit when they are used as intended, for example some over-the-counter antigen tests recommend frequent testing (or serial testing) to increase the likelihood of detecting an early infection.
As the tests are less sensitive, it is recommended that asymptomatic people who have been exposed to SARS-CoV-2 but test negative with an antigen test also receive confirmatory testing with a molecular test (described above), as antigen tests can miss infections early or late in the course of infection.
How Long Do They Take?
SARS-CoV-2 antigen tests can often return results in 15-30 minutes, and are frequently referred to in the news as the “COVID-19 rapid test”.
More information on antigen testing for COVID-19 can be found on the CDC’s website.
There are currently many ways for individuals to receive a diagnostic test for COVID-19, including at a doctor’s office, at public test sites, using an at home collection kit that is returned to a laboratory for molecular testing, and using an at home test (currently, the vast majority of at home tests are antigen tests, although moving forward at home molecular tests might become more common). Requirements at public testing sites may vary by location with some locations that require a doctor's approval prior to testing, while others, like many airport testing locations, will allow for walk up testing without prior approval. Contact your healthcare provider or visit your state, tribal, local, and territorial health department’s website to find the latest local information on testing.

Since no test is perfect, it is important to read the instructions for use to understand whether it is right for you and what to do with the result. For example, some tests are only intended for use in individuals experiencing symptoms of COVID-19, while others are intended for use in both symptomatic and asymptomatic people.
For any self-collection, it is very important to closely follow the instructions. It is also important to remember that a test is just one piece of information used to help determine whether you may be experiencing symptoms of COVID-19, or if you are contagious or shedding virus. If the test results don’t match the other information that you have or are questionable for any reason (for example, an unexpected positive test in someone with no symptoms and no known exposure), it is best to work with a healthcare provider to determine what to do next.
More information on COVID-19 diagnostic testing can be found on the CDC’s website.
Yes, it is possible for an individual to receive a negative test result even if they are infected with SARS-CoV-2 – this is referred to as a “false negative”. It is important to remember that a molecular test or an antigen test determines if a detectable level of SARS-CoV-2 is present in an individual’s sample at the time the sample was taken. Factors that impact the likelihood of receiving a false negative:
If the sample was taken too early or too late in the infection, when there is not enough viral material present, then the test result will be negative.
The accuracy of the test result relies heavily on getting a good sample from the individual.
Different tests are designed to detect SARS-CoV-2 from one or more sample type(s). Some types of samples have been proven to be excellent candidates in housing SARS-CoV-2 viral materials, including nasopharyngeal (the upper part of the throat that lies behind the nose) swabs, throat swabs, and saliva. So, if a less optimal sample type is collected, the test might come back negative even if a patient is infected.
Also, the accuracy of the test result relies on the sample being collected, stored, and handled properly! For example, nasopharyngeal swabs are difficult to perform properly, and if the swab has not been inserted deeply enough or at the correct angle, the test might report a negative result even if the patient is infected.
While tests are generally very accurate, it is important to remember that a test is just one piece of information used to help determine whether you may be sick with COVID-19. It is unlikely, but possible, for an individual to get a “false positive” result, meaning that their COVID-19 test is positive but they do not actually have a SARS-CoV-2 infection. If the test results don’t match the other information that you have or are questionable for any reason, for example an unexpected positive test in someone with no symptoms and no known exposure, it is best to work with a healthcare professional to determine what to do next, such as confirmatory testing.
As time goes on, viruses mutate (change parts of their RNA sequence). These changes or “variants” are evaluated by comparing the new sequence to the original sequence.
Testing for variants requires examining the entire RNA sequence of the virus. RNA is "sequenced" when a molecular professional uses specialized machines and laboratory techniques to “read” the bases along the RNA strand. This information is then stored in a computer file. A specialist physician or doctoral scientist analyzes the data generated through sequencing. You can read more about how DNA and RNA are sequenced on our Patient Resources for Cancer page.
Relationships to other previously sequenced SARS-CoV-2 samples can be determined by the specific combination of mutations present. Viruses with different sets of mutations may behave differently than the original virus and thus may be given a new name (for example, the Delta variant). Naming groups of viruses that share a new set of mutations allows for better tracking to see if these changes affect how vaccines and treatments work or whether the virus can spread more easily.
Variants are monitored closely by organizations such as the World Health Organization (WHO) and the United States’ Centers for Disease Control and Prevention (CDC) and may be classified as variants of interest or variants of concern. You can find further information on current variants of interest and active variant tracking on the WHO’s webpage and the CDC’s webpage.
Yes! Even though there are vaccines widely available in the US, we still need COVID-19 tests for diagnosis and for monitoring of COVID-19 levels within the population, which is also known as surveillance testing. Surveillance testing allows us to track current and new variants of SARS-CoV-2 at the population-level, and also monitor asymptomatic transmission.

Diagnostic testing remains an important tool even for those who have received the vaccine to rule out rare cases of “breakthrough” COVID-19 if they are having similar symptoms. It is important to note that at this point, these rare “breakthrough” infections are generally not causing severe symptomatic disease, but they can transmit the virus to unvaccinated children and adults. However, SARS-CoV-2 is constantly evolving, and it is possible that we may eventually encounter variants that are capable of causing severe disease in vaccinated people.
In the US, some people are not eligible or are unable to be vaccinated, while others are opting to not receive the vaccine. For these groups of people, COVID testing is especially important to identify and isolate patients that have COVID-19.
While the COVID-19 vaccine is available in the US, it is not as available in other parts of the world. Testing and contact tracing are still a major part of the public health effort in other countries where vaccines are not as readily available. Additionally, while the current rate of infection will hopefully decrease across the world, it is likely that SARS-CoV-2 will continue to circulate.
Moving forward, it will be important to use diagnostic testing to determine what type of virus is causing a person’s symptoms (for example, distinguishing between the flu and COVID-19) so that they can be treated appropriately.
When something like bacteria or a virus, such as SARS-CoV-2, enters your body, your immune system recognizes it as foreign. These invaders have unique areas on their surfaces called antigens. Antibodies are specialized, Y-shaped proteins produced by your body’s immune system that fit like a lock-and-key onto the specialized antigens on foreign invaders like a coronavirus. They are the "search" battalion of the immune system, tasked with finding an enemy and marking it for destruction. When antibodies find their target, they bind to it, which then triggers a cascade of actions that destroy the invader.

Antibody production continues until the infection is eliminated. Additionally, some antibodies can remain in your immune system after the infection is resolved. If you are exposed to that foreign substance again, those remaining antibodies can reactivate to protect you. In other words, antibodies can provide immunity, keeping you safe from another infection from that pathogen for a period of time.
For individuals who think they may have had COVID-19, or were exposed and did not develop symptoms, an antibody (serology) test can be performed. Serologic (or “antibody”) tests can detect the presence of these antibodies in blood within days, sometimes up to weeks following acute infection. These tests are not used to diagnose an active SARS-CoV-2 infection. Serologic tests can identify persons with a resolving or past SARS-CoV-2 infection, and help scientists and public health experts better understand the the risk factors and pattern of spread of COVID-19 and identify populations at higher risk of infection.
Exactly how much protection this immune response provides a person after SARS CoV-2 infection is still being studied. Until more information is known, a positive antibody test does not prove that an individual is immune from developing COVID-19 in the future, only that they were exposed to SARS-CoV-2 previously. The role of serology testing in patient care is currently being determined. For now, it is uncertain if there is a role for serology testing outside of public health monitoring as the pandemic continues to evolve.
While a COVID-19 vaccine also induces a similar, protective response from the immune system, the mechanism is slightly different. Therefore, antibody tests should not be used to determine an individual’s response to a vaccine. More information on why antibody tests should not be used to determine an individual’s response to a vaccine can be found on the FDA’s webpage.
More information on COVID-19 antibody tests can be found on the CDC’s webpage.
You can find additional up-to-date information on AMP's COVID Response Steering Committee Blog, which has kept the AMP membership and general public informed on key issues impacting molecular professionals on the front lines of the COVID-19 pandemic.
If you have ideas or requests for content in this section, we invite you to please contact us here.
Created: 09/2021 Last Updated:09/2021